polar covalent bond
A polar covalent bond is formed when atoms which are having different electronegativities and share electrons between them. A polar covalent bond is a bond formed when a shared pair of electrons are not shared equally.
 |
| Polar Covalent Bond Definition And Examples |
17 rows Bonds between carbon and other elements such as oxygen and nitrogen are polar.
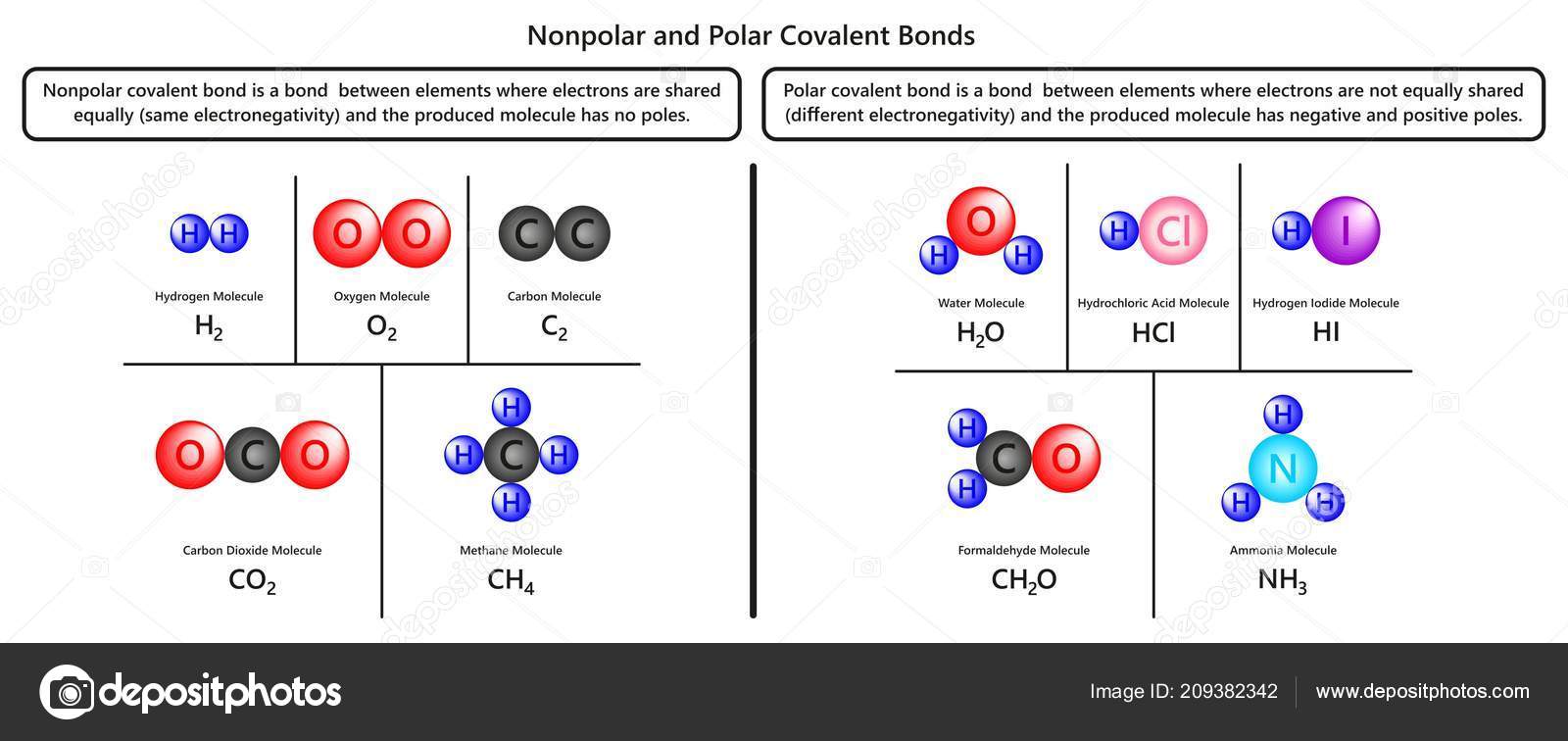
. Molecules with polar covalent bonds have a positive and negative side. So what are polar covalent bond examples. Due to this there is always an electric dipole moment in these. Polar covalent bonds result from eneven sharing of electrons.
The creation of opposing partial charges on the connected atoms known. The thief puppy has both bones ie. Polar covalent bonds can be said to be a dividing line between the formation of an ionic bond and a pure covalent bond. Polar covalent bonding is a type of chemical bond where a pair of electrons is unequally.
Typically two different elements are a large enough. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairsThe stable. A covalent bond with. Polar covalent bonds are a particular type of covalent bond.
This is due to one of the elements having a higher electronegativity than the other. In a polar covalent bond the electrons shared by the atoms spend a greater amount of time on the average closer to the. Polar covalent bonding is a chemical connection in which two atoms share a pair of electrons unequally. A non-polar covalent bond is a bond in which the electron pair is shared equally between the two.
The other puppy has lost its bone. Polar covalent bonds are made by two atoms with different electronegativities but the different should not be exceeding 17. Using periodic trends you can determine whether a molecule is polar covalently bonded or nonpolar covalently bonded. Learn how to predict if a bond will be polar or nonpolar in this video.
A covalent bond that has an unequal sharing of electrons and the electronegativity difference is within the range 01-2 is called a polar covalent bond. The polarity of. In non-polar covalent bonds electrons are. A covalent bond that.
A covalent bond with an unequal sharing of electrons and the electronegativity difference within the range of 01-2 is called a polar covalent bond. Polar covalent bonds are very common because the electronegativities of the two atoms at either end of the bond are very unlikely to be the same unless both atoms are the same.
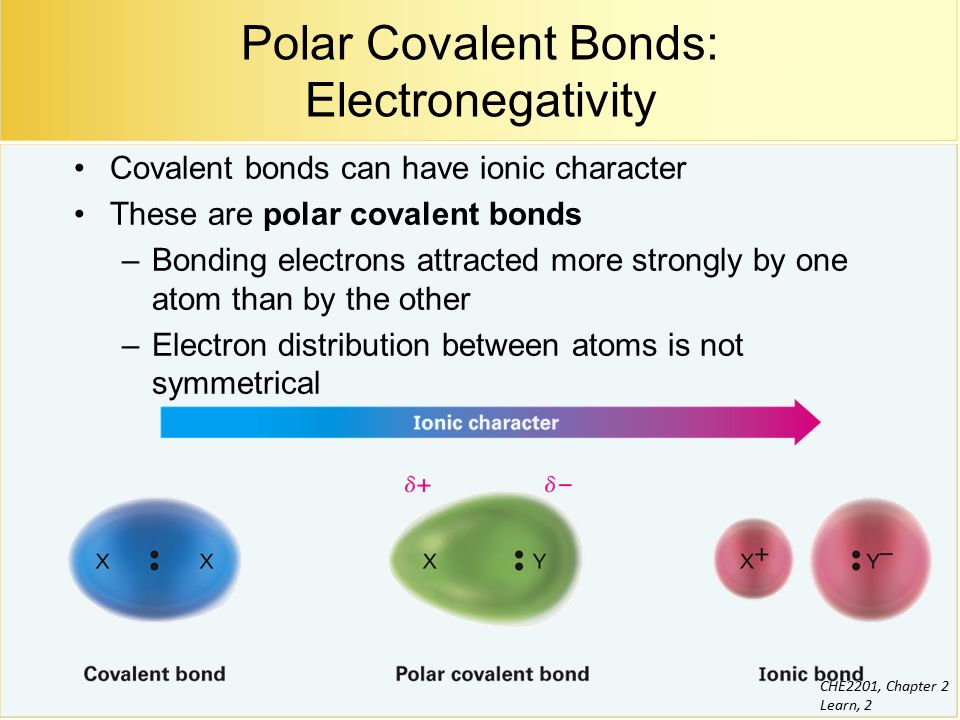 |
| Che2201 Chapter 2 Learn 1 Polar Covalent Bonds Acids And Bases Chapter 2 Suggested Problems 25 6 31 34 36 39 40 Ppt Download |
 |
| Polar Covalent Bond Polar Covalent Bond Examples |
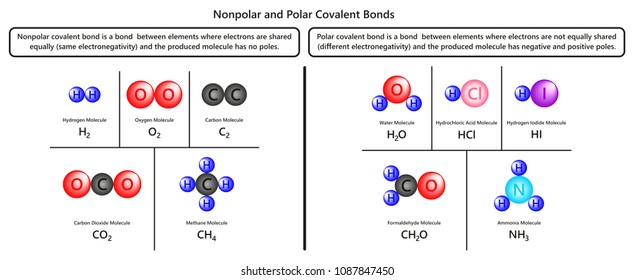 |
| Nonpolar Polar Covalent Bonds Infographic Diagram Stock Illustration 1087847450 Shutterstock |
 |
| Difference Between Polar And Nonpolar Covalent Bond Chemistry Different Types Of Covalent Bonds Youtube |
:max_bytes(150000):strip_icc()/PolarConvalentBond-58a715be3df78c345b77b57d.jpg) |
| Definition And Examples Of A Polar Bond |
Posting Komentar untuk "polar covalent bond"