bond order equation
The term bond formula refers to the bond price determination technique that involves computation of present value PV of all probable future cash flows such as coupon payments. For example in diatomic nitrogen NN the bond order is 3.
 |
| Resonance Bond Order Youtube |
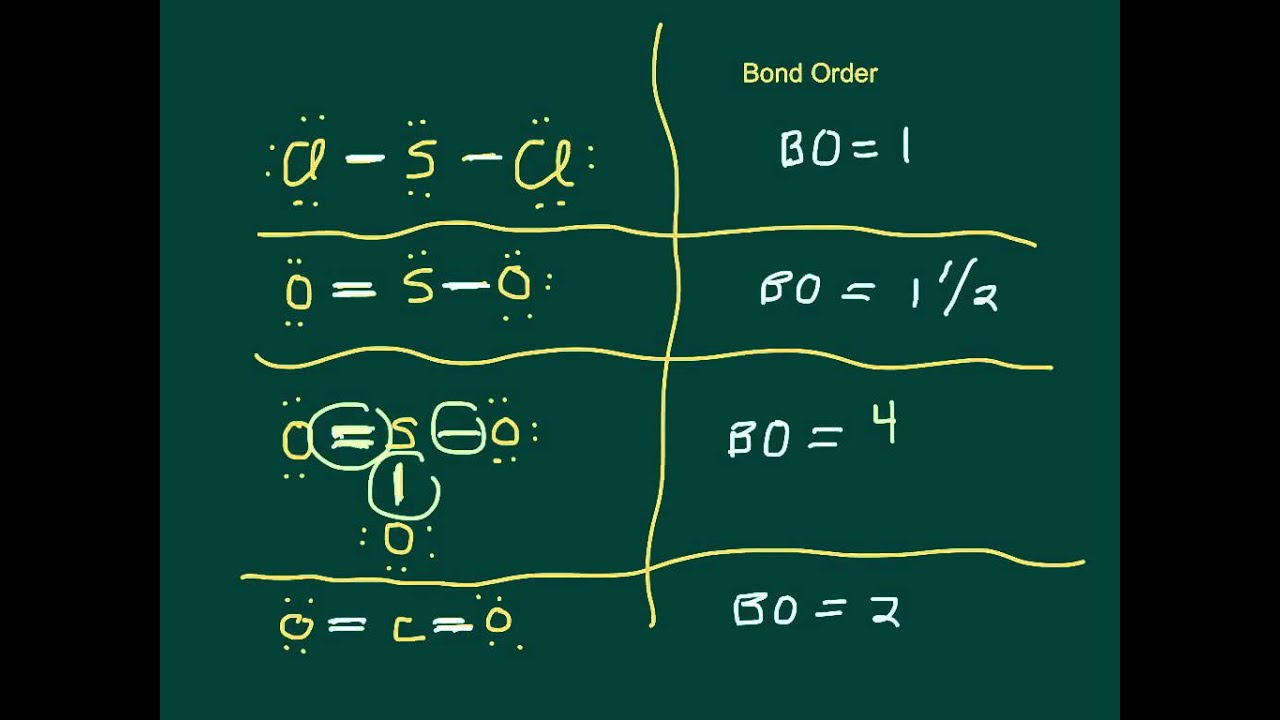
In atoms having covalent bonds for single bond bond order is 1 for double bond bond order is 2 for triple bond bond order is 3 and so on.
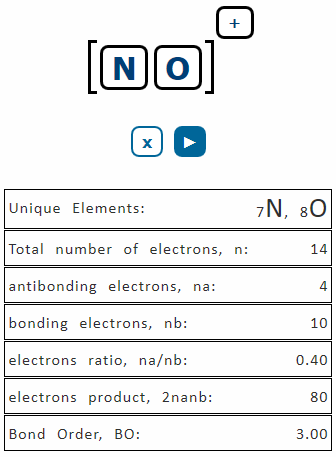
. It is defined as the difference between the electrons present in the bonding and antibonding orbitals divided by half. Formula to calculate bond order. Count the total number of bonds between the atoms concerned. Bond Order is the number of chemical bonds present between a pair of atoms is calculated using Bond Order 12Number of Bonding Electrons-Number of Antibonding ElectronsTo.
The bond order of N2 nitrogen nitrogen molecule has a triple bond so the bond order is three. The bond order formula indicates the number of chemical bonds between two atoms. Bond order formula is used to calculate the number of bonds. In molecular orbital theory bond order is defined as half the difference between the number of bonding electrons and the number of antibonding electrons as per the equation below.
Determine how many such groups of. Triple bonds have a bond order of 3. Basically bond order BO is a number of bonds present between two atoms which is measured via the electrons present in the bond formation through both covalent and ionic bonds. Bond order refers to the number of electron pairs that are shared by two atoms bonded together in a molecule.
To find the bond order of any molecule first we have. 1 2 N b N a Where Nb is the number of electrons in the bonding orbitals. And Na is the number of electrons in the antibonding orbitals. Bond order is defined as half the difference between the number of bonding and anti-bonding electrons.
BO ½ Nb Na Where Nb is the. Bond orders can be used for the analysis of the electronic structure of intermediate structures in reaction pathways. Bond order is the number of chemical bonds between a pair of atoms and indicates the stability of a bond. If the bond order of a covalent bond is 0 the two atoms in question are not covalently bonded no bond exists.
Stable bonds have a positive bond order. Bond order is calculated by. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Double bonds have a bond order of 2.
Most of the time bond order is equal to the number of bonds between two atoms. Learn the formula used to calculate bond order and see non. The Bond Order formula Bond Order number of bonding electrons - number of anti-bonding electrons 2 is used to determine the stability of a molecule or ion. Want to ace chemistry.
Exceptions occur when the molecule contains antibonding orbitals. Electronic configuration of N2. Bond Order Calculation Draw the Lewis structure of the compound or ion. Write the electron configuration of a N2 molecule.
The bond order equation is. Note that a bond is defined by the force that holds atoms together for forming molecules and. Several studies have been published in which bond orders were utilized for.
 |
| Pauling Bond Order |
 |
| What Is Bond Order Of Co Molecule Is |
 |
| Calculate The Bond Order Of Clo3 Using Resonance Concept Caution This Is Clo3 Not Clo3 |
 |
| Introduction To The Molecular Orbital Theory Mot Chemtok |
 |
| Solved Using Your Mo Diagram Of F 2 Give The Bond Order For F 2 2 F 2 F 2 F 2 And F 2 2 Which Would Have The Shortest Bond Length Course Hero |
Posting Komentar untuk "bond order equation"